AMAL Therapeutics
AMAL Therapeutics is a spin-off from the University of Geneva active in cancer immunotherapy and advancing first-in-class therapeutic cancer vaccines derived from its technology platform KISIMAÒ
At a Glance
AMAL has developed a unique protein-based immunization platform called KISIMAÒproviding activation signal to dendritic cells simultaneously to priming both helper and killer cells for various antigens and HLA restrictions. This first-in-class platform offers the opportunity to develop standardized and indication-tailored active immunotherapies.
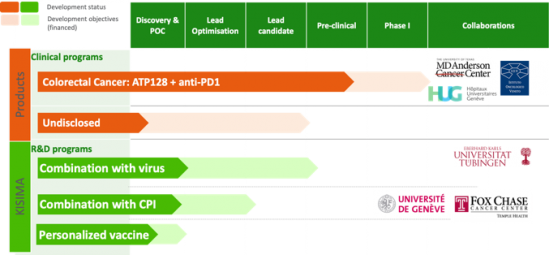
The company’s lead product ATP128 is currently being developed for stage IV colorectal cancer. The first-in-human clinical trial in combination with PD-1 blockade is planned to start in Q3 2019.
Technology Platform
AMAL Therapeutics developed a versatile and flexible recombinant protein vaccine technology platform called KISIMAÒ. KISIMA is a three-component chimeric protein with (i) a cell-penetrating peptide (CPP), a proprietary vector for antigen delivery, (ii) a toll like receptor peptide agonist as adjuvant and (iii) a multi antigenic cargo tailored to each targeted indication. This construction gives AMAL’s vaccines its unique self-adjuvancy and multi-epitopic properties. KISIMA platform also allows for simultaneous activation of both killer and helper T cells, two subsets essential for potent anti-cancer immunity. In summary, the CPP promotes the delivery of large antigens, allowing for very low injection doses (in the nmoles range) while the TLRag ensures the correct activation of all antigen presenting cells loaded with the antigens. The platform is extremely versatile, allows for repeated vaccination and can be used either as synthetic polypeptides for delivery of neoantigens or modified antigens, or as long peptide/protein with larger antigen portion carrying multiple CD8 and CD4 epitopes for various HLA restrictions.
KISIMA was established and validated in different murine tumor models along with a demonstrated link between tumor infiltration and control of tumor growth. Key parameters have been optimized including optimal dose, route of injection and vaccination schedule. A pilot non-human primate study confirmed the dose and route of injection while showing a good safety profile for the vaccine. Strong in-vivo data for combination therapies demonstrated synergies with oncolytic viruses and immune checkpoint inhibitors, which lays the basis of AMAL’s ambitious future clinical trial with ATP128.
Indication/Lead Product
Recent successes with anti-PD-1 MAbs in metastatic colorectal cancer patients with mismatch repair deficiency (MSI) generated overwhelming enthusiasm for immunotherapy. Nevertheless, patients with this cancer type represent only a small subset of the metastatic population. Current research focuses on advancing immunotherapy to earlier stages of the disease including adjuvant and first-line metastatic settings and on inducing sensitivity to immune checkpoint inhibitor therapy through a combinatorial approach. KISIMA platform offers a novel break-through pathway which creates new opportunities for immunotherapy in mismatch repair proficient patients (MSS).
AMAL’s lead vaccine ATP128 has been designed and tailored toward colorectal cancer and is being developed in combination with PD-1 blockade for the treatment of MSS patient not responding to PD-1 blockade. Such patient population represents 95% of stage IV CRC population.
The manufacturability of the compound is successfully established and process development and scale-up are completed. GLP toxicology studies are finalized and results show promising safety profile.
Pipeline